In practice, the determinations of humidity levels are carried out by drying anhydrite screeds at 40˚C. This technique is tried and proven and for that specific binder-type state of the art.The using of higher drying temperatures is refrained from, as this binder-type tends to give off more and more crystalline bound water. This crystalline bound water is from calcium sulphate and is released in two steps at 70˚C and at 105˚C (depending on the finesse of the temperature steps). In literature you find that in cementitious systems apart from anhydrite also ettringite is produced as hydrate phases.
Literature note 1:
„Diss. Christiane Rössler, 2006, F.A. Finger Institut für Baustoffkunde, Weimar“
Literature note 2:
„Diss. Severin Seifert, 2009, Friedrich-AlexanderUniversität Erlangen-Nürnberg“
Literature note 3:
„Diss. Sebastian Seufert , 2011, Friedrich-AlexanderUniversität Erlangen-Nürnberg“
Ettringite has about 50% of its molar mass bound as water, and can give off about 2/3 of it. This process can already start at lower temperatures than with anhydrite. It also depends on the humidity of the air, the higher the moisture content, the higher the temperature has to be so crystallized water can be freed.
Stepwise Drying / Kyln-drying
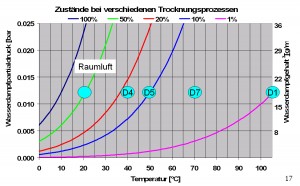
This process works at a temperature of 40˚C at a negligible extent which offers to use this temperature for the determination of free water content. The drying process is carried out up to the point of the mass constant. The height of the equilibrium is to a small part defined by the temperature and to a large part by the surrounding air humidity of an inert sample. A higher temperature leads solely to a fester reaching of the mass equilibrium. The relative humidity which has adjusted itself inside the drying closet of un-acclimatized, open systems depends solely on the surrounding air of the drying closet. The laboratory air is sucked in, heated up, and blown into the drying closet. That’s how the storage capacity of the air grows and the relative air humidity sinks, what you can easily see in the following graph. When the laboratory air of 20˚C/50%rH the relative air humidity (equilibrium humidity) in the drying cabinet at about 40˚C is less than 20%rH. A sample, which is at equilibrium in that climate, can be called very dry.
Stepwise, drying, frame/surrounding conditions
Dry screeds
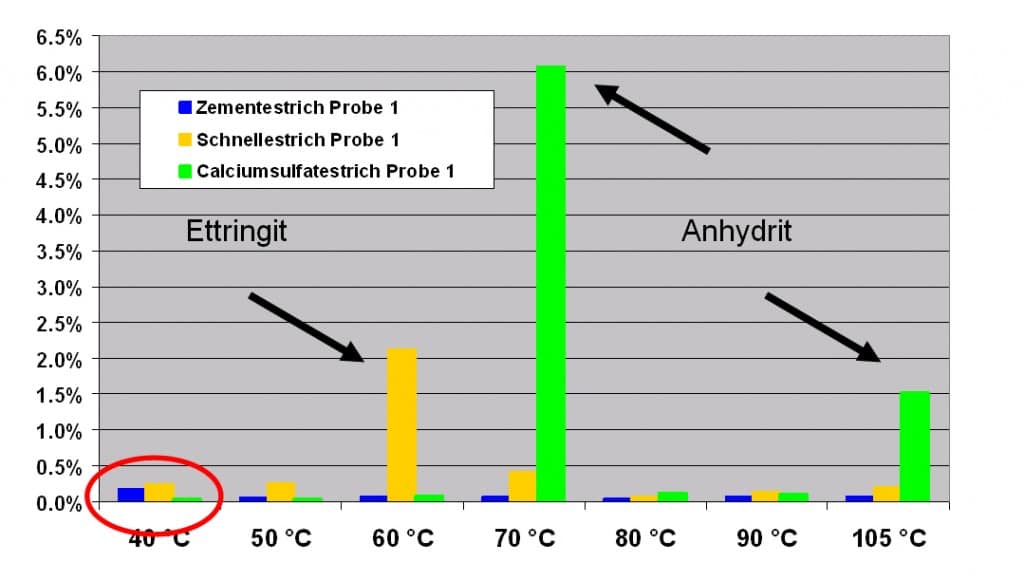
Using the step wise drying process, a sample’s loss of weight can be determined using given temperatures and the relative humidity of the air. In addition to the amount of free water (weight loss at 40˚C) you can also determine characteristic traits of cementitious samples (which the following graph shows using three different samples) when using different drying temperatures. Looking at the graph we can for example establish, that the yellow sample has to be a quick setting screed while the green sample is a conventional calcium sulphate anhydrite screed (CA). The blue sample is a cement swab. The graph shows that in all samples there is only a slight humidity loss at 40˚C and therefore they only contain a small amount of free water.
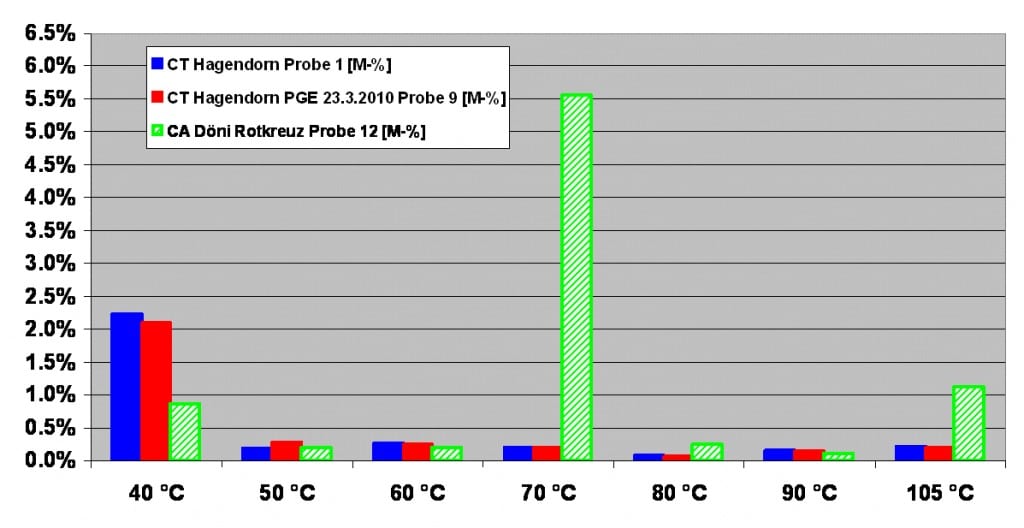
Wet screeds
Stepwise drying of 3 humid screed samples. When fresh samples of a site are being dried stepwise, a completely new picture of the weight loss at 40˚C is created, like the following graph shows.
Stepwise drying of 3 humid screed samples,
At 40˚C the 3 samples show a high weight loss, and therefore allow us to determine the amount of free water in a sample. The green sample can be characterised as CA looking at the high, characteristic weight loss at 70˚C, while the other samples are classic cementitious screeds.
Filler
Fillers which also contain a large amount of ettringite and anhydrite can be tested using the same procedure and can be characterised with only little technical effort. Following, we have added a graph with respective filler samples.
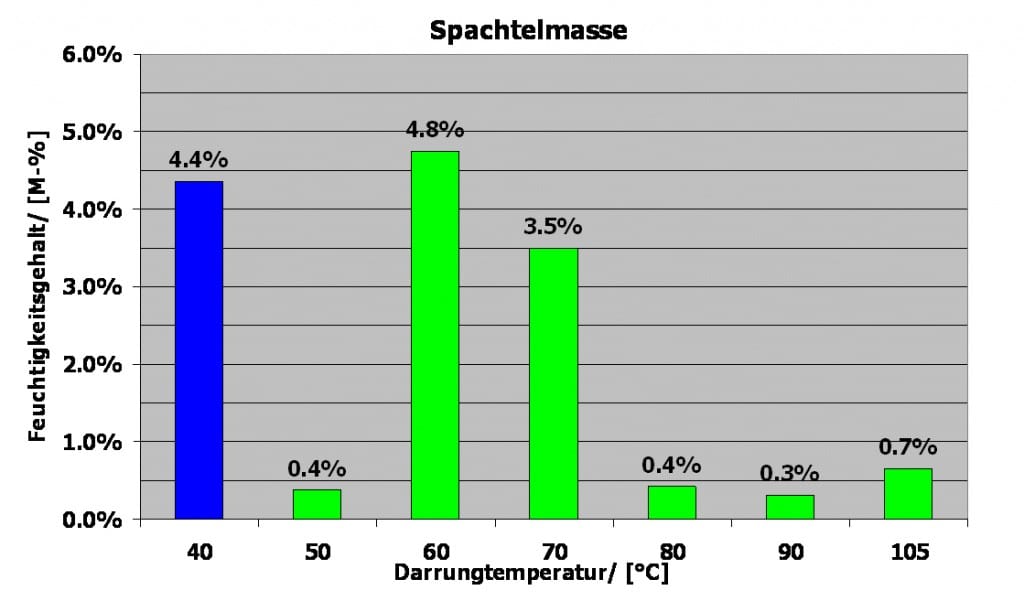
Stepwise drying of filler
The results definitely show that the sample, even though having lost a large amount of weight at 40˚C, it still held a large amount of free water. The extreme weight loss at 60˚C shows that the sample contains a large share of ettringite, while the weight loss at 70˚C can be ascribed to the anhydrite.
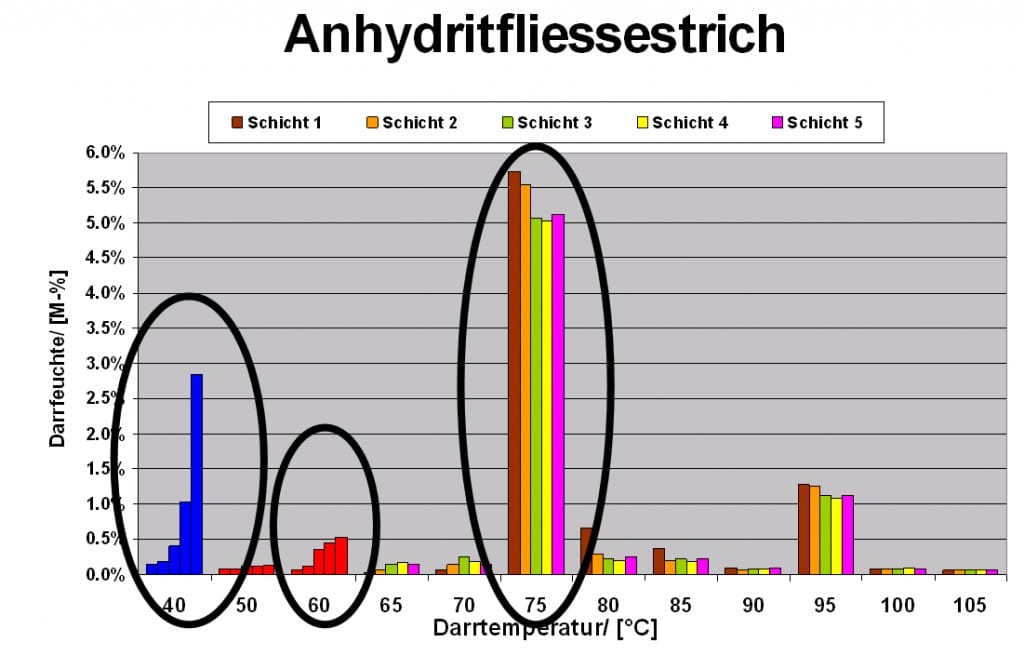
Humidity gradient of a Calcium sulphate-tile screed
When the sample gets taken properly, you can get a proper humidity profile for the extraction point even with stepwise drying. The compositions of the different weight loss points show the clear layers over the whole cross-section. CAF contains apart from anhydrite filler always a certain part of cementitious components arranging the setting behaviour. This test is used to determine the Drying temperature up to 60˚C in 10˚C steps while they are being carried out in steps of 5˚C. Through very different amounts of weight loss at 40˚C, we can guess what humidity gradient runs through the screed construction. You can clearly see that the ettringite share above the cross-section isn’t regular, and that there is a lot more anhydrite further above than there is below.
Closing statement
Even though this whole system is rather simple, even from a technical point of view, it has a major disadvantage.
A characterisation as clear as this one needs a couple of weeks. For every chosen temperature, a sample has to be dried to its weight equilibrium. A termination criterion was, as it’s explained in Norm DIN 18121, a weight loss of less than 1 M-% in less than 24 hours. Good things need time…..


Write a Comment