Basics / History
The carbide method describes the reaction of calcium carbide with water to produce calcium hydroxide and acetylene. Water, in every state of matter, in combination with calcium carbide is used to create the white, fine, powdery calcium hydroxide and the gaseous acetylene. The formed amount of gas can be easily determined by measuring the increase in pressure. Due to this reaction the moisture content can be easily determined in any given sample. Historically, this method for the determination of water content was first mentioned in 1906 by P.V. Dupré. (P.V. Dupré, The Analyst Vol. XXXI., No.364, July 1906)
Precise Measurements
The moisture measurement with the carbide method counts, due to it being highly precise, as an analytical measuring procedure analogue to the humidity measurements according to the Karl Fischer Titration or the drying cabinet procedure. As water is used up during the reaction, each sample gets dried out. The state of equilibrium where can be reached when the residual partial water pressure lies at around 10 -10 mbar! In its state of equilibrium no more acetylene gas is produced, and the forwards and back reactions are equally fast. A successful reaction of carbide and water requires that both reacting agents are in contact with each other. The actual rate of reaction of water and carbide to acetylene and calcium hydroxide is extremely fast. The more porous a sample is, the bigger the influence of mass transfer (where the water molecules are the mobile reaction partner) on the overall reaction rate
Applications with Reactions to Completion
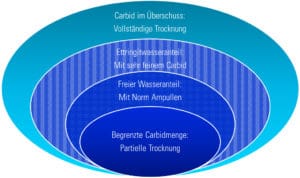
The moisture measurement with the carbide method always leads to a complete drying out of the sample if the carbide is present in excess compared to the amount of water, and the reaction is given enough time to complete (it is also important that there is no obstruction of the transport of water). If there is less carbide than water present, then the drying process will only be completed partially and there will be residual water left in the sample. In both cases the reaction can reach its state of equilibrium. These reactions are used to, for example, determine the moisture content of bulk materials according to the norms of DIN or ASTM.
Applications with a Premature Termination of a Reaction
The time it takes for the reaction to complete depends on the mobility of the water as well as the available area of contact of the carbide. This specific measurement procedure can be used, for example, to determine the free water content for the testing of the readiness of screeds according to different norms of DIN, SIA, UNI or to determine the amount of ettringite found in a sample. The testing for the readiness of screeds of mineral building materials are undertaken according to the DIN 18560, the SIA 252 or the UNI 10329 measurement procedure. Through maximisation of the rate of reaction, crystalline bound water in a binded structure, such as ettringite in a ternary binder system, can also be determined. In both of these applications, the measurement is stopped before the chemical reaction has a chance to be complete. When used like this, the measurements are terminated bevor the chemical reaction is completed.
In our blog you can find complementary articles about the moisture measurement using the carbide method as well as further techniques for moisture measurements.
Image 1: Equation of reaction for the moisture measurement with the Carbide-Method
CaC2 + 2H2O -> Ca(OH)2 + C2H2
Carbidesolid+Water->Calciumhydroxidesolid+Acetylenegas
Image 2: Depending on the amount of Carbide a sample can be dried completely.
Image 3: Over excess of Carbide each sample will be dried - even a slice of apple!